Has cuffless blood pressure been proven possible?
August 2025 , Sam Moreland
Has Hilo (previously Aktiia) cracked cuffless blood pressure? Well they’ve got FDA clearance so that must be true right?
As with anything it's not quite as simple as that and for me the jury is still out because there seems to be a big hole in their validation studies. Now I may be wrong and its just that the information is not publicly available yet, so I’m happy to amend this if the evidence changes. But as of right now there is a huge problem.
Now I’m going to caveat everything that I’m about to say with for a single site cuffless blood pressure measurement through PPG, the method Hilo is using is likely the only way it could work.
Ok so what does Hilo do. Technically they don’t measure blood pressure but change in blood pressure. Here's how it works:
They take a calibration measurement with a manual BP cuff which they use to calibrate the algorithm (remember this). They use this measurement as a baseline to measure the changes from. So they don’t actually measure the absolute underlying blood pressure.
They analyse the PPG pulse wave for indications of change in arterial stiffness which is highly correlated (but not exactly) with BP.
Ok so foundationaly it seems ok, so what's the issue with the FDA clearance?
Please note I will interchangeably use Hilo and Aktiia as they are the same company, but the clinical validation literature refers to them as Aktiia.
FDA Clearence
I’ve looked at the FDA summary, the clinical trial proposal and the predicate devices and it's a bit murky on what they’ve actually really tested. The main sticking point is how the calibration measurement is used. The trial proposal doesn’t actually give the protocol of how they combine the calibration measurement for the algo and testing. They've also not released a validation study paper describing it which I’d like to see.
I wrote a blog post on the difference between cuff based and non-cuff based which goes in great depth on the issue. From it I discuss the foundational difference between cuff based measurements and non-cuff based. Cuff based systems impose external pressure onto the arteries, thereby being able to make a measurement, the theory goes like this:
1. Increase the cuff pressure until blood stops flowing through it. At this point the external pressure is roughly equal to the maximum blood pressure (systolic) because blood can’t push through.
2. You decrease the pressure in the cuff gradually, during this period, not all blood flows through the cuff and flows in a different way (turbulent flow).
3. When the external cuff pressure decreases to the point where blood flows through naturally (laminar flow) we call this the diastolic pressure.
For cuff-based BP validation they use the ISO 81060-2 protocol, which requires you to compare against a reference measurement (nurse auscultation). To get an accurate comparison, the protocol requires you to keep the participant hemodynamically stable to minimise change in BP between when the cuff and reference measurement is taken (so you measure the same thing). In order to validate that it can measure over a range of BPs, you get people with lots of different pressures rather than vary a single participants BP (as its hard to keep stable).
Hilo and other single-site cuffless BP does not measure absolute pressure, but uses a calibration measurement with a separate cuff-based BP monitor, to get absolute pressure and the cuffless device “measures” a change in BP. So depending on how you perform the reference measurement, plays a huge role in following the ISO 81060-2 protocol. For example, you take a calibration measurement, keep the patient hemodynamically stable, then produce a cuffless blood pressure measurement and a reference measurement. There has been no change in BP in the participant so you could just reproduce the calibration measurement as your algorithm and you could be accurate (depending on how accurate your calibration cuff is). This is why ISO 81060-3 exists and should be the only way devices like Hilo should be able to be cleared by the FDA for BP measurement.
Now before I continue there needs to be a bit of information on BP.
What is the point of long term BP trend monitoring?
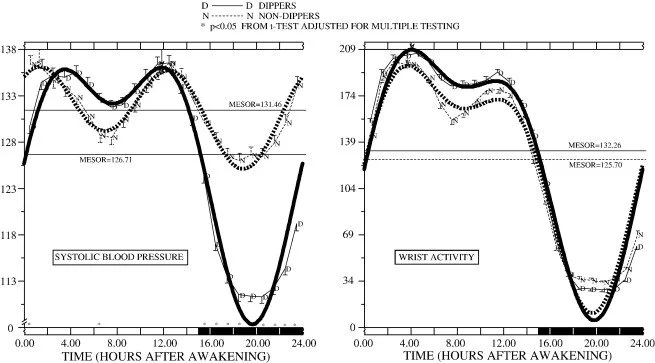
We all know our BP changes. Just standing up will raise your systolic by 10 mmHg, just as doing meditation can lower it. But what doctors look for is not your environmental reaction to BP, but your baseline diurnal (changes in the day and night), specifically they will look for the following trend.
Diurnal BP changes, Hermida et al, Circadian variation of blood pressure: The basis for the chronotherapy of hypertension, 2007
The image above shows the BP differences between day and night. Specifically when you go to sleep you expect your BP to “dip” when you wake your BP to “surge” (with some variance among meal times). Deviations for this pattern are what blood pressure specialists look for to tell them what type of hypertension you may have and the best method of treating. From Hermida et al:
“Subjects with a diminished nocturnal BP decline (non-dipper pattern) have a significantly worse prognosis than the ones with a normal dipper pattern. In particular, the non-dipper circadian BP pattern represents a risk factor for left ventricular hypertrophy, microalbuminuria, cerebrovascular disease, congestive heart failure, vascular dementia and myocardial infarction.”
So being able to classify dipping is incredibly important and it's what hypertensions specialists need to see. Normal dipping is around 10-20% of your daytime BP. There are also weekly variances as well but it's less well understood in a clinical setting.
The problem with Hilo's clinical trials.
Hilo's own trials
Their own published papers unfortunately do not really give any good reassurance for the accuracy and applicability of the device. Hilo/Aktiia have done two types of study, the ISO 81060-2 standard trial design (used for FDA) and a long term test against 24 hour Ambulatory Blood Pressure (ABP). 24 hour ABP is where you wear a traditional cuff based device and it automatically inflates at set periods so you don’t have to do it manually.
Now unsurprisingly their previous ISO 81060-2 standard trials get similar results to the one they passed for the FDA, however the long term studies are very interesting and I think misleading for any actual useful clinical or personal use of the device.
I will caveat with the fact that comparing against 24 hr ABP is difficult because the 24 hour ABP has its own error rates, so is not a perfect reference point for direct comparison, but in reality it is the only way beyond arterial lines which may not be ethical.
Their Jan 2025 validation paper was done against 24 ABP on 63 patients age 53.1 ± 7.2 years, 21.2% female . I will only be taking the main aspects of it so please read the full paper to draw your own conclusions.
In this study they compare the Aktiia (Hilo) wearable against the changes seen against the 24 hour readings captured from the ABP, but the statistical methodology for analysing it is very interesting:
“Average 24-h, daytime (9am-9pm) and night-time (11 p.m.−7a .m.) systolic and diastolic BP (SBP, DBP) and heart rate (HR) were calculated for 1-day ABPM and for 7-day Aktiia monitor, at the beginning and at the end of the CR program. The 7-day average for the Aktiia monitor provides a comprehensive representation of BP for one week in the lives of the patients.”
Now this method is very confusing on a few levels. For a start a 7-day average does not provide a comprehensive representation of BP for patients if you're claiming ABP like functionality. Daily averages have some applications, but not 7 days (also in the paper they mix 7 day and 1 day averaging and it's difficult to follow what is actually being compared).
Second is the averaging of the data. The Hilo device can measure spot instances of blood pressure, and the ABP device measures spot instances of data. A better comparison would be a direct pairwise regression, possibly with averaging measurements before and after the ABP measurement to balance out any irregularities. Possibly this is done because of the errors associated with the ABP, but this isn’t stated in the paper and to me is a bit unconvincing.
Now the important part of the paper is that they specifically look for the dipping and diurnal trends.
“Average daytime (9 a.m.−9 p.m.) and night-time (11 p.m.−7 a.m.) SBP and DBP were calculated for 1-day ABPM coinciding with 1-day Aktiia monitor, at the beginning and at the end of the CR program. For each patient, measurements were organized in two sessions of paired data. In this case, session 1 compared first day's ABPM and Aktiia data; session 2 compared final day's readings from both devices.”
So for diurnal comparison they average over specific time windows. Now these windows are fixed which is usually done in 24 hour BP analysis, but it lends itself to contamination between windows when different people wake and sleep at different times. This contamination effectively smooths out the differences and would give better results to devices that would not follow dipping at night very well, especially if the sample sizes are small compared to the magnitude of change (this information is not provided).
So what did they find?
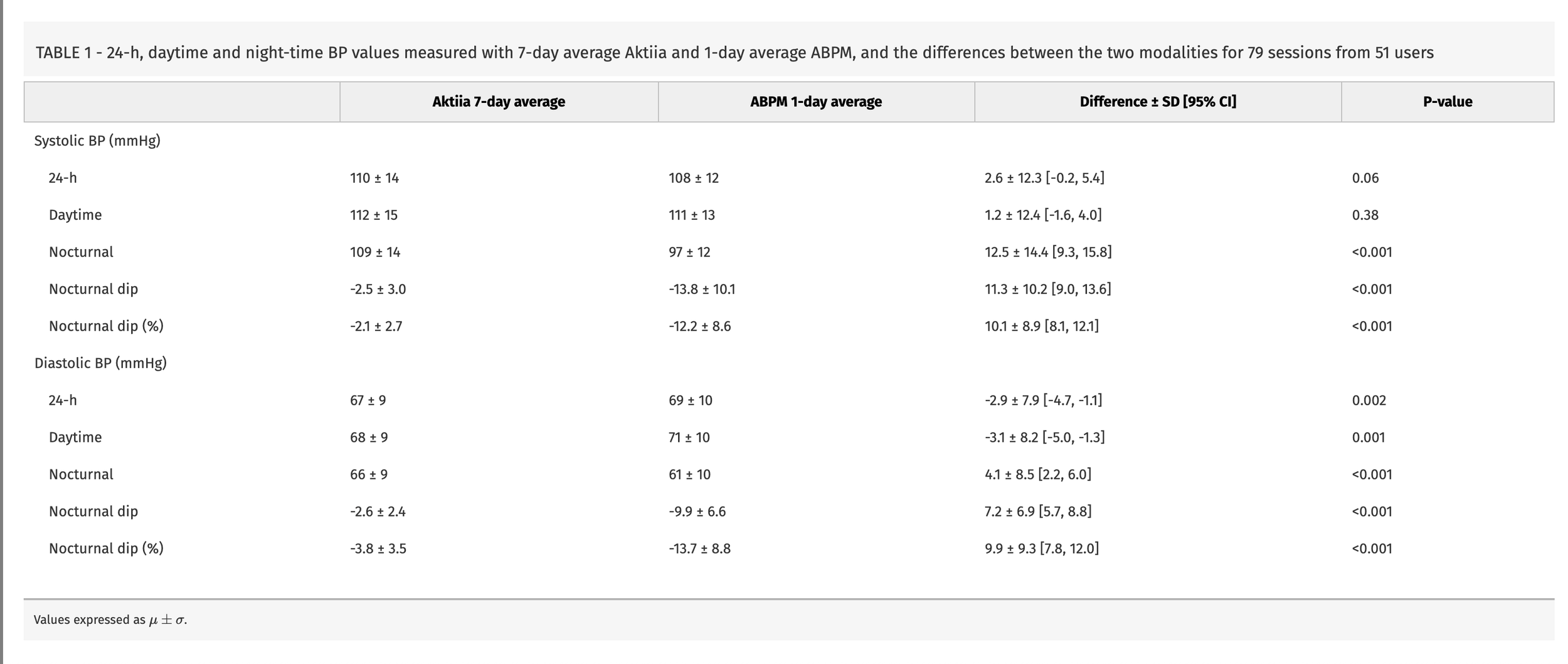
Dirunal BP accuracies between Aktiia and 24 hour ABP, Almeida et al., Evaluation of Aktiia cuffless blood pressure monitor across 24-h, daytime, and night-time measurements versus ambulatory monitoring: a prospective, single-centre observational study, 2025
They find a large difference in nocturnal measurements of 12.5 +- 14.4 mmHg SBP and 7.2 +-6.9 mmHg DBP. To put this in perspective your night time dip is around 10-20%, so this error is a substantial overestimate in reference to BP values. If your SBP is 120 mmHg you could expect a 12 - 24 mmHg drop. The daytime agreements are quite strong but the std differences are substantial. They also do some statistical wrangling to make an argument for being able to detect dippers based on a ROC curve, but it seems like they're just fitting the data to the narrative they want. As it is a change in BP that Hilo/Aktiia are claiming to be able to measure, this study is pretty bad, even given the errors associated with ABP there should still be decent change and agreement.
But the conclusions they draw from the study are interesting and it feels like we’ve been reading 2 different papers.
“The present clinical trial has shown that one week of Aktiia monitoring compares well to 24-h of ABPM monitoring.”
And
“As a comparative study, it is essential to recognize that if the metrics used in the analyses do not align perfectly, it does not inherently imply that the devices are unreliable for individual subjects. Instead, it suggests that the devices offer alternative characterizations of the same individuals, both of which contribute valuable information. Importantly, the Aktiia monitor and ABPM utilize distinct methods to measure BP, which naturally lead to variations in BP readings under various conditions [19]. Firstly, ABPM readings taken with oscillometric cuffs are influenced by hydrostatic and gravitational forces that change with the body's position [20]. In contrast, the proprietary algorithm used in Aktiia devices has shown resilience to such positional changes [12]. Secondly, from a metrological standpoint, current validation protocols for oscillometric cuffs do not evaluate their performance on different body positions, particularly when the subject is lying down during sleep [17]. Consequently, while recent data underscored the importance of BP monitoring with ABPM [4], it is essential to interpret oscillometric cuff data with caution when obtained in positions other than sitting. In comparison, Aktiia has shown consistent BP measurements across various body positions, including supine, when compared to auscultatory BP readings [12].”
Now for a start the whole reason to do 24 hour ABP is to look and plot the diurnal differences of spot measurements. Hilo here have averaged measurements and their disagreements are massive, showing that they do not follow night time dips. This is not a good 24 hour ABP device.
Secondly , it suggests that the devices offer “alternative characterizations of the same individuals, both of which contribute valuable information”. What is this information? How is it different and how does this information have any clinical meaning or effect on patient outcomes.
Third they note “ABPM readings taken with oscillometric cuffs are influenced by hydrostatic and gravitational forces that change with the body's position. In contrast, the proprietary algorithm used in Aktiia devices has shown resilience to such positional changes”. Yes, your blood pressure changes with position and BP cuffs measure that. The fact that Aktiia doesn’t measure it is not a good thing, it shows that they are not actually measuring blood pressure. I’m not sure why this is a positive?
All in all this paper shows that the ability for Hilo to be a like for like comparison of a 24 ABP in its actual clinical use case is low. It also has a lot of statistical wrangling and marketing in it that make it a confusing read and they take liberties in presenting their device in the most flattering light.
But it's not the only paper that has looked at Aktiia/Hilo on diurnal trends…
Non-Aktiia Papers
Tan et al. did a similar test to the Hilo paper. However they did a pairwise spot check basis rather than an average and they also looked at tracking medication induced changes (it was also a better paper to read).
They found an overestimation of 15 mmHg SBP and 11 mmHg DBP at night (similar to Hilo). Not only this but they tested the watches ability to track BP changes from medication and found that it could not. Their conclusion was:
“We showed that despite comparable daytime BP, the cuffless method did not adequately track the BP decline during night-time sleep when compared to conventional cuff-based ABPM. Our study results also suggested that the cuffless device did not adequately track antihypertensive medication-induced BP lowering when compared with cuff-based HBPM.”
and
“The commercially available wrist-worn Aktiia cuffless wearable device lacked the key function required for clinical management, namely the ability to track changes in brachial BP during sleep or with drug treatment in a similar fashion as conventional cuff-based methods. While awaiting a new ISO standard which is specific for validating cuffless devices, studies are encouraged to evaluate the ability of wearable devices to track changes in BP, as opposed to just validating the devices at resting or calibration BP.”
Which for me hits the nail on the head with the issue of the clearance of Hilo and for future non-cuff based devices.
What's missing?
So in both papers (and other Aktiia papers), they discuss the daytime BP as being pretty accurate. Well there's actually a crucial piece of information that is not included in either of the studies; how variable were the BP readings during the day? If not very much, then the algorithm doesn’t have to do very much and in fact its best bet is to stay closer to the calibration measurement (like in the ISO standard).
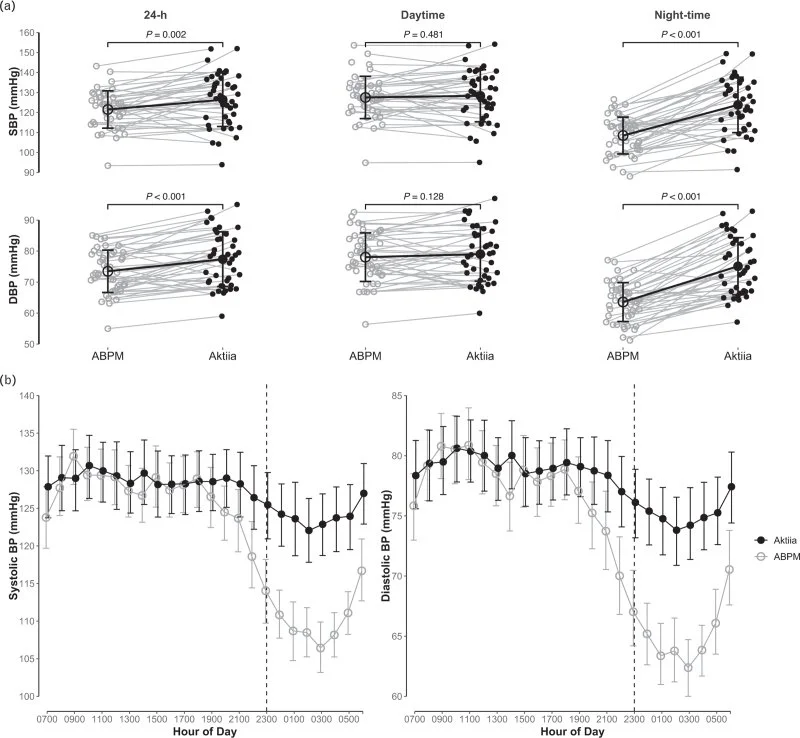
Well that data is unavailable. Both studies focus on the difference between the Hilo and the ABP devices. But what is interesting is looking at the diurnal plots from Tan et al.
From this plot it looks like the daytime BP does not vary a great deal until around 17:00 - 19:00 at which point the Aktiia device no longer follows it. What is interesting is the consistent after lunch dip which is correctly tracked by the ABP device is not by the Aktiia. Given the confidence intervals its not super cut and dry, but it does seem that daytime BP variance is pretty low.
Other irregularities
There are a few other things around the company that make me question the device.
Why not use the ISO 81060‑3:2022 standard for measuring continuous blood pressure. This is most equivalent to what Hilo are actually measuring (change in blood pressure).
In all of Hilo's studies up until the FDA study, they only required once a week calibration claiming this was all that was necessary. But for the FDA submission this is changed to once a day. Why not calibrate 6 days a go and then do the study?
Why change the name to Hilo shortly before your FDA submission (under the name of Aktiia) comes out. Especially with 120,000 units already sold there's brand recognition. People who google for “Hilo device accuracy” without knowing their previous name would not be able to find the validations showing poor diurnal capabilities.
They have just raised a $42 million Series B, so there are investor pressures.
Why has it taken 20 years?
All of these could just be moot but they could be important in understanding the current way Hilo are working.
What can we conclude from this?
This is a lot less cut and dry than the Whoop debacle. Aktiia/Hilo on the surface have been somewhat transparent with their studies, but they draw conclusions and massage the information in a way to make them seem comparable to a traditional cuff based device when they are not.
And without more information on the validation study, my conclusion is that Hilo (and in future others) have found an exploit in the ISO 81060-2 BP validation standard (also thought by Tan et al.). They take a calibration measurement in the same test conditions as the validation reference with little to no BP change. This makes it super easy for their algorithm to weigh that heavily. You are essentially testing whether or not the device remembers reference BP measurement.
In comparison with the long term 2 clinical studies that showed that Hilo could not follow BP changes at night or follow medication induced BP changes. Also the usage of averaged and manipulated data in the Hilo study suggests they are trying to show their product in the best light and not give good comparisons against actually clinically useful products. The fact the BP during the day does not vary too much also makes it suspicious for claims it is accurate during the day. From these studies it shows clearly the device does not track BP changes which is what they claim in the FDA summary.
Combined with my own experience with PPG signals I have huge doubts the information they portend to get from the waveform to estimate BP is simply not there, especially at the wrist site. This means that regulators seriously need to look at what these watch based devices are doing. They could be giving patients dramatically wrong measurements of their BP which can lead to serious consequences!
Now I’m happy for Hilo to reach out to clarify things I may be missing or publish their validation study for the FDA, but until that happens you should not buy this watch to monitor your BP.